Dies ist eine alte Version des Dokuments!
Crocus sativus L. - Iridaceae - saffron, saffron crocus, Safran, Echter Safran, Gewürzsafran
Perennial without above-ground stem, unknown as a wild plant; leaves 2-4, linear, with a white midrib, 8-15cm long, appearing in autumn with the lilac-purple flowers; anthers yellow, filaments purple; style red, branched, up to 3cm long; fruit 3-celled capsule.
„Cultivated in the main Kashmir valley. Being a triploid it is sterile and propagated by vegetative means only. Known only under cultivation; Saffron, obtained from long red style branches and stigmas are in great demand for eastern culinary preparations and in medicine. It is rich in vitamin B2.“ http://www.efloras.org/florataxon.aspx?flora_id=5&taxon_id=200028148
The carotenoid crocin is primarily responsible for the color of saffron.
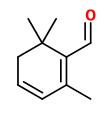
4-Hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde is a precursor of safranal and may be obtained by mild extraction methods like ultrasound-assisted extraction in amounts of 0.4mg/g saffron (safranal: 6mg/g).
[Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde (HTCC) in Greek saffron. Kanakis, C. D., Daferera, D. J., Tarantilis, P. A., & Polissiou, M. G., Journal of agricultural and food chemistry, Vol.52(14), 2004, 4515-4521]
„Safranal, the main component of Crocus sativus essential oil, is thought to be the main cause of saffron unique odor… Beside its exclusive color, saffron presents a particular taste which is originates from its picrocrocin content. Safranal (2,6,6-trimethyl-1,3-cyclohexadien-1-carboxaldehyde) is a cyclical terpenic aldehyde produced from picrocrocin. Picrocrocin, discovered by Kajser (1884), cracks down following acids and alkali conditions, resulting in a molecule of water and an aglycon which in turn, loses a water molecule and finally turns to safranal. Kuhn and Winterstein (1933), who obtained safranal through picrocrocin hydrolyzation for the first time, named this chemical as Safranal.“
[Safranal: From an Aromatic Natural Product to a Rewarding Pharmacological Agent. Ramin Rezaee1 and Hossein Hosseinzadeh, Iran J Basic Med Sci. Jan 2013; 16(1), 2013, 12–26]
Aroma extract dilution analysis (AEDA) was used for the determination of aroma-active compounds of Iranian saffron obtained by solvent-assisted flavour evaporation (SAFE). „According to sensory analysis, the
aromatic extract obtained by SAFE was the most representative of saffron odour…. A total of nine aroma-active compounds were detected in the aromatic extract. On the basis of the flavour dilution (FD) factor, the most powerful aroma active compounds were safranal (FD = 512), 4-ketoisophorone (FD = 256) and dihydrooxophorone (FD = 128).“
[GC-MS-olfactometric characterization of the most aroma-active components in a representative aromatic extract from Iranian saffron (Crocus sativus L.)., Amanpour, A., Sonmezdag, A.S., Kelebek, H., Selli, S., Food chemistry, 182, 2015, 251-256]

Köhler,F.E., Medizinal Pflanzen, vol.2 t.164 (1890)
http://plantgenera.org/species.php?id_species=288759